Description
【Main Composition】
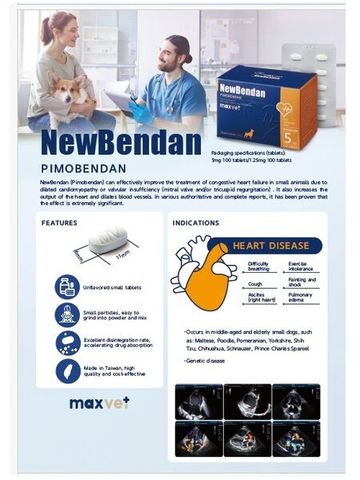
Each New Bendan tablet contains 5.0 mg of pimobendan
【Indication】
Dog: For the treatment of congestive heart failure due to dilated cardiomyopathy, or valvular insufficiency (mitral valve and/or tricuspid regurgitation). (Also refer to the package leaflet under “Application and Dosage") Used in the treatment for Doberman Pinschers where this diagnosis using cardiac ultrasound detected a preclinical dilated cardiomyopathy (a case where there is an increase in the left ventricular end-systole and end-diastolic diameters, however no symptoms appeared.)
【Species】Dogs
【Dosage and Administration】
Before commencing treatment, an accurate weight needs to be measured to calculate the correct dosage. NewBendan should only be taken orally, and the dosage taken should be within the range of 0.2 mg to 0.6 mg of pimobendan per kilogram of body weight per day, and divided into two portions per day. The ideal daily dosage should be 0.5 mg per kilogram of body weight, divided into 2 dosages (i.e. 0.25 mg per kilogram of body weight per dose) and should be administered 1 hour before the morning and evening meals. For dogs weighing 20 kg, administer one tablet in the morning and the other half in the evening. The medication can be used in combination with diuretics, such as furosemide etc.
【Warning】
1. This product cannot be used for hypertrophic cardiomyopathy or for those dogs suffering from functional or structural reasons that cause an inability to strengthen cardio output (such as aortic stenosis).
2. Pimobendan is metabolized mainly be the liver, hence, it should not be used for patients with severe liver dysfunction.
(Please also refer to this leaflet under “Recommendation for pregnancy and breastfeeding.”)
3. Do not exceed the recommended dosage. On the basis of the dog’s weight difference, the tablet can be halved at the indentation to ensure accuracy of dosage.
4. This medication is to be used by the owner, livestock and poultry breeders, mariculturists or feed mills in accordance to the veterinarian’s prescription.
【Storage Conditions】
Please keep out of reach of children. Do not store above 25 ° C. Please store this medicine in the original packaging box. Keep it airtight at all times to avoid moisture.
The expiry date is indicated on the box and on the bottle. Do not consume after expiration. After opening, please consume within 100 days. Halved tablet should be consumed at the next dosing interval.
【Adverse Reactions】
It is rare for an ill dog to elicit a positively chronotropic effect and vomiting, but these responses are dose-dependent and can be improved by lowering the dosage.
It is rare for a dog to have a temporary diarrhea, lack of appetite or energy.
Although the relationship with pimobandan is not clear, in very rare patients it may be observed during treatment that primary coagulation function is affected (mucosal purpura, subcutaneous hemorrhage), and these symptoms disappear after stopping the drug. It is rare for patients to have a long-term use of pimobendan in the treatment of mitral valve disease, an increase in mitral valve regurgitation.
The frequency, terminology and definition of adverse reactions occurring are as follows:
Extremely common: 1 out of 10 animals having adverse reactions during the course of the treatment Rare: more than 1 out of 10000 animals, but less than 10.
Common: more than one out of 100 animals, but less than 10 Extremely rare: not even reaching 1 out of 10000 animals, including case reports.
Uncommon: more than 1 out of 1000 animals, but less than 10.
※ If you find any serious adverse reactions or other reactions not mentioned on the package information, please contact your veterinarian immediately.
【Special Warning】
This product has not been tested on those Doberman Pinschers with asymptomatic dilated cardiomyopathy that also has combined atrial fibrillation or persistent ventricular tachycardia
【Warnings for use on animals】
The blood sugar level of the dogs with diabetes should be tested regularly during the treatment period. When used in preclinical dilated cardiomyopathy (increase in the left ventricular end-systolic and end-diastolic diameter, but no symptoms have occurred), a comprehensive cardiac examination should be performed (including cardiac ultrasound, if possible, conduct Holter monitoring). Monitoring of the cardiac function is recommended during treatment with pimobendan.
【Warnings for operators】
Wash hands after handling this medicine. If accidentally ingested, please bring this package leaflet and label to the doctor immediately.
Suggestions for medical treatment: Ingestion of this medicine, especially by children, can initiate tachycardia, postural hypotension, facial flushing and headache.
【Recommendations for pregnancy and breastfeeding】
Experiments of laboratory rats and rabbits did not find side effects such as teratogenic or foetotoxic effects. However, at high dosage, maternal poisoning and embryotoxic reactions may occur, and also that pimobendan was found to be secreted into the milk. There wasn’t any evaluation conducted on the safety of pregnant and lactating female dogs, so the dosage can only be determined by the judgement of the veterinarian on the treatment benefit and risk assessment.
【Drug Interaction】
In the pharmacological study, there was no interaction effect between pimobendan and cardiac glycoside strophanthin. However, the increase in contractile force induced by pimobendan can be attenuated by the following drugs: calcium antagonist verapamil, diltiazem, and β sympathetic antagonist propranolol.
【Overdose】
Overdose may result in tachycardia and vomiting. Symptoms should be treated and dosage lowered. After the healthy beagles were given a dosage that is 3-5 times higher of the recommended dose for an extended period (6 months), some developed a thickening of the bicuspid valve and left ventricle, a change caused by pharmacodynamics.
【Waste Treatment】
Unused medicines or empty bottles should be disposed of in accordance with the waste disposal regulations.
Related information:
This product can be taken in conjunction with furosemide for dogs with symptomatic valve regurgitation and can effectively improve the quality of life and to prolong life.
This product can be taken in conjunction with furosemide, enalapril and digoxin for dogs with symptomatic dilated cardiomyopathy (limited number of dogs experimented), and can effectively improve their quality of life and prolong life.
In a randomized, placebo-controlled trial; the test dogs included Doberman breeds with preclinical dilated cardiomyopathy (diagnosed by cardiac ultrasound, increased left ventricular end-systolic and end-diastolic diameter, but no symptoms). When used pimobendan, it can delay the time of congestive heart failure or of sudden death occurring and can prolong life.
In addition, using pimobendan in the treatment of preclinical dilated cardiomyopathy can effectively reduce the size of the heart of the dog. The efficacy evaluation was based on 2 trials. The data of 19 (39 total) dogs and 25 (37 total) dogs reached the primary clinical efficacy index. The experimental group was not those treated with the pimobendan group or the placebo group.
Package
10 tablets/box, 100 tablets/box, 500 tablets/bottle
- Pimobendan 5mg
- congestive heart failure
- cardiomyopathy
- valvular insufficiency
Production Capacity:
Not informed
Delivery Timeframe:
Not informed
Incoterms:
Not informedPackaging Details:
Not informed
More about
Yumax / Maxvet Inc
10-50
Employees
2M - 10M
Sales volume (USD)
10%
% Export sales
Year
Established
Business type
- Importer / Trading Company
- Distributor / Wholesaler
- Retailer
Keywords
- Pimobendan
- Maropitant
- Apoquel
- Onsior
- Telmisartan
- Vetoryl
- Benazepril. Ver Mais
Contact and location
-
David ********
-
+886 2********
-
Taipei / nil | Taiwan